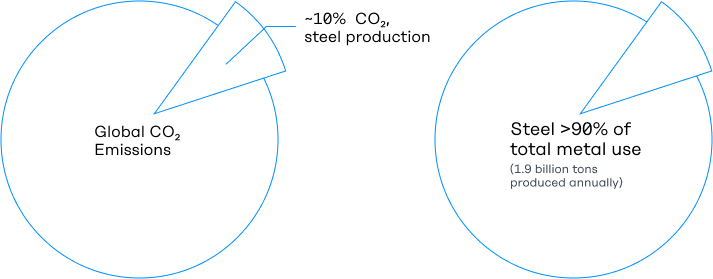
Metals are among our most important engineering materials, and steel is their undisputed king. Steel production accounts for over 90% of total metal use, with nearly 1.9 billion tons produced annually. It is the second most-produced engineered material after cement.
Steel is strong, tough, heat-resistant, and easy to work with, making it ideal for various applications. It’s also cheap, with strength- and toughness-to-cost ratios that are unparalleled. Integrating steel into a myriad of applications is rational and, in many cases, the only choice.
But this miraculous combination of material properties and cost comes with a significant challenge—at ~3.6 Gt per year of CO2e, steel production is responsible for nearly 10% of global CO2 emissions.
Where do steel emissions come from?
Emissions in steelmaking come from the energy used to heat, melt, and form the material and direct process emissions from the use of reducing agents. Reducing agents are substances that are used to reduce iron oxide to iron metal. Since process emissions are a major part of GHG emissions in steelmaking, just decarbonizing energy – although very important – won’t be enough to achieve net zero in this sector.
Steel is an alloy of iron and carbon. Iron rarely occurs in pure form in nature – most abundantly an oxide, iron oxide must be reduced to iron metal, separated from the other components of the ore, and alloyed with carbon to produce steel. We have manufactured steel in essentially the same way for over 3000 years, leveraging carbon as the reducing agent. Carbon reacts with iron oxide to form steel and carbon oxides.
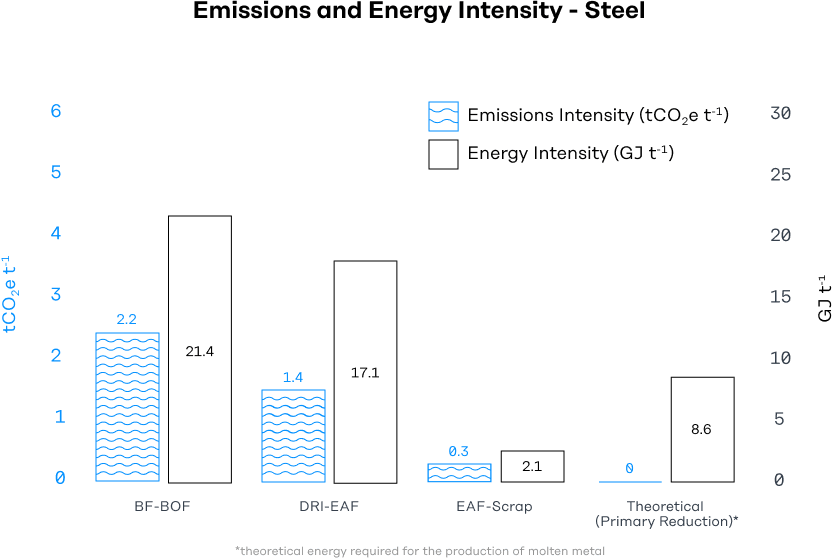
Today, steel is made in 3 primary ways. Over 70% of steel production uses blast furnaces with coal-derived met coke. Nearly 90% of steel is recycled at end-of-life, and so-called secondary steel makes up about 24% of global production via Electric Arc Furnaces (EAF). Direct Reduced Iron (DRI), using natural gas or coal-derived syngas, accounts for the remaining ~5%.
The below graph displays energy intensity, the amount of energy it takes to produce one ton of steel, and emissions intensity, the amount of CO2-equivalent emissions that are produced by making one ton of steel.
Why steel is a hard-to-abate sector
Steel is foundational to our civilization and demand for steel is projected to increase through the 21st century. China is the #1 steel producer, accounting for over half of the total production, followed by the EU, India, Japan, the USA, Russia, and South Korea. Steel is one of the most challenging to decarbonize industries because:
- It is low cost ($400-800/t for crude steel)
- Metals have unique material properties that are highly desirable for a wide range of applications, and steel is already the lowest-emissions structural metal that we make
- A large fraction of steel’s emissions are due to process emissions from the chemistry of the reducing agents used in the steelmaking process
- We have millennia of experience making and using steel, and iron is the most abundant element on Earth
Pathways to address GHG emissions in the steel industry
Alternative materials
Alternatives to steel exist for certain applications, such as mass timber in construction and aluminum in automobiles and aircraft. Unfortunately, steel has one of the lowest emissions per unit mass of any of our structural materials. There aren’t many materials that we currently produce that provide a decarbonization benefit when compared to steel (on an embodied carbon basis).
Steel use extension
It is possible to reduce the amount of steel required in a given application through more efficient manufacturing (e.g. additive manufacturing) or through strength enhancement (e.g. superalloys, grain structure).
Secondary steel is vital to global production and is the least energy- and emissions-intensive steelmaking process. Although most steel is recycled, managing impurities like copper and phosphorus is challenging. Solutions for impurity separation are crucial to maintaining and increasing the production of secondary steel.
Decarbonized energy for steel production
In addition to the energy required for reduction (which is supplied by reducing agents) existing steel manufacturing processes use significant energy in the form of heat and electricity to heat and melt ores and metal and to cast/roll/finish steel into final form. Decarbonizing the electricity of EAF secondary steel production could nearly eliminate the carbon footprint of this process. Decarbonizing the non-reduction energy required to operate blast furnaces and DRI plants could reduce the emissions intensity of these processes.
There are further opportunities to improve the efficiency of existing processes, but these are incremental because blast furnaces and DRI plants are mature processes that have already been optimized for efficiency
Alternative reducing agents in existing processes
It is possible to use reducing agents other than coal or methane in conventional processes.
Biomass-Derived Carbon
The use of biogenic, rather than fossil, carbon as the reducing agent can eliminate net emissions. Innovations in process design as well as biomass feedstock aggregation are required for this to be a scalable, cost-effective solution.
Alternative sources of carbon can be integrated into the blast furnace at up to ~20% (or in some cases up to 50% if meeting purity requirements), and this approach has already been demonstrated by incumbent steel producers.
Hydrogen
Hydrogen DRI swaps out the fossil-derived syngas in conventional DRI for clean hydrogen, building off decades of development in the DRI steelmaking process. Despite lower energy use and emissions, global DRI production is less than 10% of blast furnace steelmaking due to its reliance on high-purity ores, as it lacks a slagging process for separating iron from ore impurities. Expanding DRI use will require additional separation processes, either upstream (ore beneficiation) or downstream (post-reduction slagging).
Hydrogen currently costs significantly more than natural gas on an energy basis. Although hydrogen DRI is a possible way to reduce emissions, it costs more than conventional steelmaking unless we can find innovations to reduce hydrogen costs and unlock the use of lower-grade ores.
New ore beneficiation and separation solutions can benefit steelmaking pathways beyond DRI by reducing the cost, energy requirements, and emissions of steelmaking. They could also enable new reduction technologies.
Hydrogen can also be incorporated into blast furnace steelmaking as a supplementary reducing agent.
Carbon Capture and Sequestration (CCS)
Adding CCS to a steel plant requires additional energy for CO2 separation from the exhaust gas and significant capital expense. At an average of 2.7 tons of CO2e per ton of steel produced by a blast furnace, carbon capture at ~$100/ton CO2 would increase the cost of steel production by 30-70%.
New reduction pathways
In addition to using new reducing agents in existing processes, there are potential innovations that leverage new pathways for achieving reduction and/or separation.
Electricity
One of the few technologies capable of substantially improving the efficiency of the steelmaking process while eliminating its carbon footprint is electrochemistry.
Boston Metal uses a high-temperature process (Molten Oxide Electrolysis) to make molten iron directly from broad classes of ore feedstocks with electricity as the reducing agent. Separation is achieved in this slagging process in analogy to conventional blast furnaces. This solution allows for the direct integration of Boston Metal plants into conventional steelmaking processes.
Electra uses a low-temperature electrochemical reduction process to produce 99% pure iron that can be directly integrated into the steel supply chain via EAFs. Their solution allows for flexible inputs including the use of intermittent renewable energy sources and a wide variety of ores as feedstock, including low-grade ores, because of a chemical separation process that precedes the electrolysis.
Electrochemical systems are inherently modular. In order for these solutions to compete on cost with conventional processes, their capex and the cost of clean energy must be comparable to incumbent solutions.
Heat
Iron oxide can be reduced at very high temperatures without additional reducing agents. Even a partial reduction with heat can reduce the requirement for reducing agents. However, no practical system to achieve the high temperatures and separation required for full direct thermal reduction has yet been proposed. There are suggestions of using lasers or microwaves to directly heat iron ore particles, but these have yet to demonstrate sufficient efficiency or cost viability. Low-cost thermal decomposition solutions are an area of active exploration.
Metals
Metallothermic reduction is used in the production of many high-value metals. This technique works by using a different metal as a reducing agent, ripping the oxygen off of iron ore. While this approach is technically valid, it typically has a much higher cost than steel production can sustain. This cost comes from the capex of metallothermic reactors, the opex required to separate the products of the reaction, and the inefficiency of recycling the metal used as a reducing agent, which inevitably is a more valuable metal than steel.
Biogenic Carbon
Biogenic carbon can be used in novel reduction processes that do not require the same mechanical properties as modern blast furnaces. In addition to challenges of cost and efficiency when compared to modern blast furnaces, the use of biogenic carbon requires massive aggregation of biomass.
Hydrogen
Alternative, non-DRI reduction pathways leveraging hydrogen as the reducing agent have been proposed. Potential advantages of these systems include the ability to separate the sensible heat requirements of the process from the reduction energy (i.e. only using hydrogen for reduction, not for heating) and the opportunity to integrate a slagging event into the process to enable the use of lower-grade ores. Most proposed solutions are still in the early stages of development
Global steel production is partitioned according to the least-cost method of production. The availability of ores/feedstocks, the cost and availability of different sources of energy, and the cost of transportation of ore and produced metal, along with the policy/regulatory framework and cost of capital, determine the relative cost of different steel production technologies for a given geography. The declining cost of renewables, innovations in steel production and mining, as well as the evolving policy landscape will reshape the global steel market in the coming decades.
We have solutions that are deployable today, but to fully decarbonize steel, we need continued research and development and a holistic policy approach to create and scale cost-competitive options with minimal green premiums.